

1. Clearly Define Cases Involving Criminal-Administrative Enforcement Linkages

Preface
On May 9, 2025, the State Administration for Market Regulation released the "Guidelines for the Supervision of Medical Advertisements" (hereinafter referred to as the "Guidelines"), which establish a regulatory framework for medical ads featuring tiered oversight, four categories of prohibitions, and collaborative governance. Building on an interpretation of these guidelines, this article will analyze relevant cases of administrative penalties imposed on medical advertising and offer practical compliance recommendations.
I. Key Takeaways from the Guide
(1) Tiered Regulation: Three Categories Under the Principle of Proportionality Between Penalty and Offense
|
Circumstances Not Subject to Administrative Penalty |
★Only basic medical information (such as name, address, category, etc.) is published and must be accurate; ★Ad content remains unchanged but was published despite the review documents having expired; ★The advertisement content falls within the scope of legally required public information and is supported by evidence. |
|
Circumstances for lighter, reduced, or no punishment |
★Promoting accurate information about the same medical consortium or its internal members; ★ Real-life images shown are accurate. Location details are genuine and precise; ★ Introduce standardized and precise terminology for approved subjects and their scope; ★Information presented is based on official documents and does not involve efficacy or technical details. |
|
Aggravated penalty circumstances |
★ Making definitive claims about efficacy and safety, or promoting cure rates or effectiveness rates (subject to enforcement under the Advertising Law); ★Special Focus: Major and complex diseases such as cancer and myopia prevention among adolescents will be punished more severely according to the law. |
(II) Four Types of Prohibitions: The Four Major Ban on Medical Advertisements
Prohibition of medical beauty advertising targeting minors: Advertising for medical beauty procedures not intended for treating medical conditions—particularly those that could harm minors' health—is strictly forbidden through mass media or channels accessible to minors.
Ban on Beauty-Related Anxiety-Making Medical Aesthetic Ads: Medical aesthetic advertisements that promote or exploit concerns about physical appearance—violating societal norms of decency—are prohibited.
Prohibit the use of absolute language: Medical advertisements are strictly forbidden from using absolute terms such as "best" or "highest-grade" (to be handled according to relevant enforcement guidelines).
Prohibition of Endorser Recommendations: Endorsers are not allowed to promote or vouch for medical services (in violation of the ban on medical advertising).
(III) Collaborative Governance: Coordination Between Criminal Enforcement and Platform Accountability
1. Clearly Define Cases Involving Criminal-Administrative Enforcement衔接
(1) Fabricating or falsely using the names of patients, doctors, institutions, etc., to provide testimonials or endorsements;
(2) Fabricating or distorting scientific theory data to claim efficacy/safety or guarantee cure rates/effectiveness;
(3) Misleading key information—such as affiliation, ratings, and technology—of fictitious institutions can distort patients' healthcare choices.
According to the relevant judicial interpretation [1] If the above-mentioned circumstances constitute false advertising and involve any of the following scenarios, public security authorities shall initiate criminal investigations: (1) The amount of illegal gains exceeds 100,000 yuan; (2) The direct economic loss caused to a single consumer is over 50,000 yuan, or the cumulative direct economic loss to multiple consumers exceeds 200,000 yuan; (3) False advertising is conducted under the guise of preventing or controlling emergencies, deceiving numerous individuals and resulting in illegal gains of more than 30,000 yuan; (4) Although the aforementioned monetary thresholds are not met, the entity has already received administrative penalties for engaging in false advertising twice within the past two years and continues to do so; (5) Physical injuries or disabilities are caused as a result; (6) Other circumstances deemed particularly serious.
2. Strengthen the principal responsibility of advertising platforms
Advertising platforms must rigorously review the qualifications of healthcare service providers and related self-media accounts listed on their platforms. Platforms found to have failed in their verification duties—or those that have provided services enabling medical advertisements to be published by non-medical institutions or individuals—will be investigated and penalized according to law. In severe cases, these platforms will be ordered to suspend operations for rectification, ensuring that advertising platforms fulfill their "gatekeeper" responsibilities.
II. Empirical Case Study from a Guidebook Perspective
(1) Big Data Analysis Based on 100 Administrative Penalties for Illegal Medical Advertisements [2]
1. Common Types of Violations
Based on our analysis and summary of the sample cases, it’s evident that in many instances, a single medical advertisement involved multiple violations. Guideline Article 16 clearly states: If a single medical advertisement exhibits multiple types of violations, but these violations constitute a **single** unlawful act, the principle of prohibiting repeated penalties applies. However, if the violations amount to **multiple independent offenses**, they should be jointly penalized according to law.
|
Type of Violation |
Proportion |
For example |
|
Publishing medical advertisements without censorship |
80% |
Advertising is published without obtaining the "Medical Advertising Review Certificate" or with content that does not match the approved certificate. |
|
Using prohibited content |
30% |
Using patient testimonials or endorsements, featuring spokespeople to make recommendations, highlighting medical technologies, guaranteed cure rates or efficacy, employing absolute language, and targeting beauty-related ads that play on appearance anxiety—among other practices. |
|
Non-medical institutions publishing medical advertisements |
10% |
Non-medical entities such as cultural companies, wellness centers, equipment firms, handicraft businesses, and individuals are prohibited from publishing medical advertisements that promote disease treatments or use medical terminology. |
|
Necessary information not labeled |
5% |
Necessary information such as the name of the medical institution and the review registration number is missing. |
|
False or Misleading Advertising |
10% |
False advertising of medical technologies, pharmaceutical products, or their efficacy—either directly or disguised as science-based or health-related content intended to promote medical ads. |
2. Common Institutions and Channels Featuring Illegal Medical Advertisements
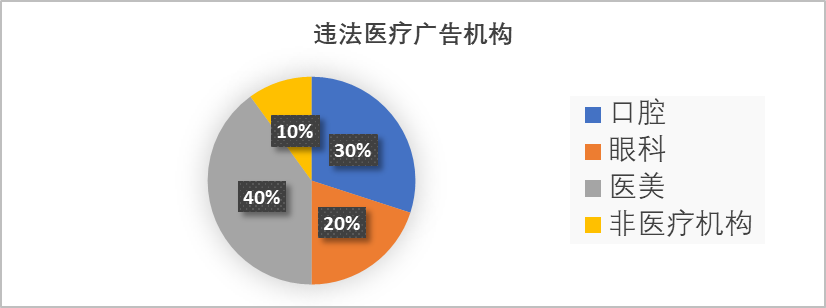
Figure 1: Statistical Chart of Illegal Medical Advertising Agencies
Medical aesthetics and dental advertising are high-risk areas for illegal medical ads, with non-medical institutions—including various cultural companies, wellness centers, medical device firms, health supplement companies, and even individual practitioners—commonly involved.
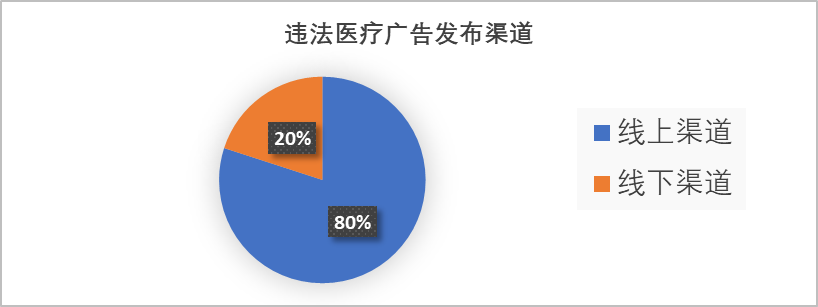
Figure 2: Chart Showing Distribution Channels of Illegal Medical Advertisements
The tech-neutral defense is invalid: Programmatic push cannot exempt platforms from their obligation to conduct manual content review.
Social Harm Assessment: Linking dental aesthetics to marriage, romance, and career prospects constitutes psychological pressure that violates the ethical底线 of medical practice.
3. Analysis of Penalty Outcomes
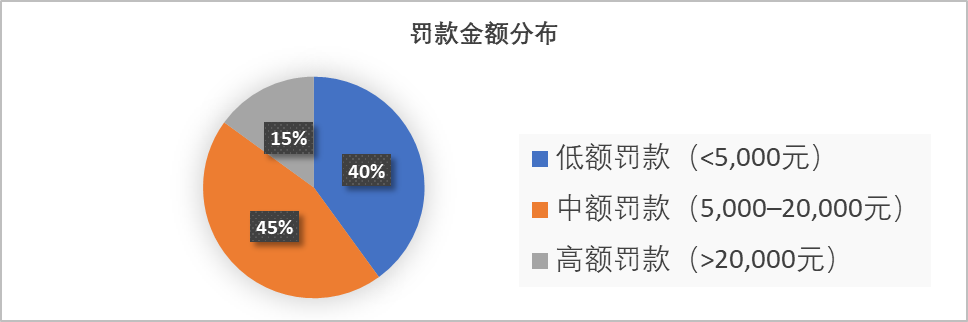
Figure 3: Chart Showing Statistics on Fines for Illegal Medical Advertisements
Most of the fines imposed for illegal medical advertising fall into the medium- and low-range categories, with only a small number of significantly larger administrative penalties. [3] The administrative penalty hearing process must be followed. Local standards for determining larger amounts subject to administrative penalties vary significantly—for instance, the threshold for fines imposed on corporations or organizations stands at over 100,000 yuan in Guangdong Province, more than 50,000 yuan in Shanghai, and above 20,000 yuan in Jiangxi Province. Medical institutions should pay close attention to local regulations and ensure they fully understand the potential implications of hearing procedures that may arise from administrative penalties related to medical advertising. In cases involving false advertising or repeated violations, the situation could even meet the criteria for criminal investigation (refer to the "Coordination Between Administrative and Criminal Enforcement" section mentioned earlier).
(II) Typical Administrative Litigation Cases Involving Medical Advertising
Based on the key points of the guidelines, the author has selected several representative administrative litigation cases involving medical advertising—specifically those addressing prohibitions against absolute terms in medical ads, the responsibilities of platforms publishing medical advertisements, administrative penalty hearing procedures, the ban on creating appearance-related anxiety, and the principle of proportionality in penalties. Below is a summary of the case details, along with the court’s key rulings and compliance insights.
|
Basic Facts of the Case |
Laws and regulations |
Key Ruling Points and Compliance Insights |
|
In March 2020 to May 2021, a hospital in Gansu faced a case of misleading medical advertising. The hospital claimed on its official website that it offered "the largest number of gene-testing projects and the fastest report turnaround time in the province," while its official WeChat account used phrases like "world's most advanced pathology equipment." Meanwhile, promotional materials posted on the hospital’s public account stated, "China sees 130,000 new cases of cervical cancer each year," but failed to cite the source of this statistic. After being reported, the hospital removed the controversial content but did not revise its historical web pages. As a result, the market regulatory authorities imposed a fine of 300,000 yuan. Dissatisfied with the penalty, the hospital subsequently filed an administrative lawsuit in court, which ultimately upheld the original fine of 300,000 yuan. |
Article 9 of the Advertising Law (Prohibition of Absolute Terms) Article 11 (Data Must Include Source Attribution) Article 28 (Criteria for Determining False Advertising) |
The use of absolute terms like "maximum" or "fastest" is deemed misleading unless certified by an authoritative body, as it constitutes false advertising. Data citation rule: Even if the data is accurate (according to National Cancer Center data), failing to cite the source is illegal. Ongoing Violation Status: If the historical page is not deleted, it will be considered a continuous publication of illegal advertisements. |
|
In the Shenzhen LocalBao Technology-Baidu Alliance case, in September 2020, Shenzhen LocalBao, a local information platform, automatically pushed ads for "Dr. Miao's Acne Treatment" through the Baidu Alliance. These ads contained misleading claims such as "cure acne completely" and "consultation by top-tier hospital experts," which were later reported as false. The platform initially claimed it was unaware of the content being promoted. Following the incident, Shenzhen LocalBao filed an administrative lawsuit. In the first-instance ruling (by the Yantian Court), the court overturned the Luohu Administration for Market Regulation’s decision to impose an 80,000 yuan fine, citing insufficient evidence to prove that the platform knowingly published the false content. However, in the second-instance judgment (delivered by the Shenzhen Intermediate Court), the court ordered the Luohu Administration for Market Regulation to reconsider the case and ultimately reduced the penalty to 30,000 yuan. The court reasoned that the platform had failed to fulfill its obligation to filter keywords—specifically, it did not block terms like "cure completely"—and noted that the ad-generated revenue was minimal (only 824 yuan) and that this was the platform’s first offense. |
Article 28 of the Advertising Law (Liability for False Advertising) Article 12 of the "Administrative Measures for Internet Advertising" (Platform Review Obligations) |
Platform Duty of Care: The platform bears the responsibility for conducting a formal review of the advertiser's qualifications (e.g., the medical institution's practice license). Please note: Medical terms such as "radical cure," which are explicitly prohibited, clearly constitute illegal practices and thus place the platform on notice. The tech-neutral defense is invalid: Programmatic推送 cannot exempt platforms from their obligation for manual content review. |
|
In the Huai'an Happiness Hospital case, in April 2021, the hospital involved used pre- and post-operative comparison photos of patients on bus stop signs and claimed on its website to possess "Korean-style seamless double-eyelid patent technology" (which, in reality, was not patented). After being reported, the market regulatory authorities imposed an immediate fine of 400,000 yuan without holding a hearing. The hospital subsequently appealed, filing an administrative lawsuit. The court ruled that the city’s market supervision bureau had violated procedural regulations during the administrative penalty process, leading to the annulment of the original penalty. As a result, the bureau was ordered to reissue the penalty, this time reducing the fine to 150,000 yuan. |
Article 16 of the Advertising Law (Prohibition on Using Patient Images for Endorsement) Article 63 of the Administrative Penalty Law (Large-Fine Cases Require a Hearing) |
Procedure for Illegal Determination: A fine exceeding 50,000 yuan (the higher threshold standard in Jiangsu Province) requires notification of the right to a hearing; investigation records are invalid if signed by only one law enforcement officer. Evidence exclusion rule: Patient photos obtained through procedurally unlawful means cannot be used as the basis for a final decision. |
|
In the Hainan Yijia Medical Aesthetics case involving appearance-related anxiety, in November 2020, the hospital implicated in the incident posted an advertisement on its Meituan store that read: "Yellow teeth → Rejection in love → Setbacks at work," leveraging concerns about physical appearance to promote its teeth-whitening service. The ad drew complaints, prompting the market regulatory authorities to impose a fine of 150,000 yuan on the hospital. Dissatisfied with the penalty, the hospital filed a lawsuit in court, which ultimately upheld the original fine of 150,000 yuan. |
Article 9 of the Advertising Law (Violation of Social Morality) "Article 3 of the Enforcement Guidelines for Medical Aesthetic Advertising (Prohibition of Creating Appearance Anxiety)" |
Application of Retroactive Legal Effect: The advertisement was published in 2020, while the guidelines came into effect in 2021. However, the legal principle of "public order and good morals" can be applied retroactively. Social Harm Assessment: Linking dental aesthetics to marriage, romance, and career prospects constitutes psychological pressure that violates the ethical底线 of medical practice. |
|
In the Renqiu Zhongyuan Dental case involving absolute advertising claims, in March 2022, the hospital was found to have displayed promotional banners within its premises that read, "The Most Advanced Technology for Dental Implants" and "The Best Method for Missing Tooth Restoration," among other slogans. After being reported, the market regulatory authorities imposed the maximum penalty of 7,500 yuan—five times the advertising costs. However, the hospital later filed an administrative lawsuit, contesting the decision. Following a court review, the penalty was reduced to 3,000 yuan—equivalent to twice the actual advertising expenses. |
Article 9 of the Advertising Law (Prohibition of Absolute Terms) Article 5 of the Administrative Penalty Law (Principle of Proportionality Between Offense and Penalty) |
Exception to the rule: "Dental implants are the best method for restoring missing teeth" is a medically recognized consensus (supported by evidence cited in the textbook *Oral Implantology*), and thus will not be penalized as commercial hype. Discretionary Penalty Adjustment: Since the advertisement was displayed only within the premises, reaching a limited audience, and this is the first violation with immediate corrective action taken, the fine amount has been reduced. |
III. Compliance in Medical Advertising and the Establishment of a Compliance System for Medical Institutions
Medical advertising compliance is a critical component of compliance efforts for healthcare institutions. Based on the guidelines, the author offers the following recommendations for ensuring medical advertising compliance and building robust compliance systems within healthcare organizations:
(1) Key Considerations for Compliance with Medical Advertising
1. Prior Review and Qualification Management
★ Mandatory application for the "Medical Advertising Review Certificate"; submit your application to the provincial Health Commission or Traditional Chinese Medicine Administration before publication. The certificate is valid for 1 year and must be reapplied for upon expiration or in case of changes to practice information (e.g., addition or reduction of clinical specialties).
★The ad content must strictly match the approved proof. Published materials must be identical to the authorized sample—no cutting, splicing, or modifications are allowed. Additionally, the ad must clearly display the medical institution’s official first name and the review approval code.
2. Strictly adhere to the "negative list" for advertising content
(1) Only eight types of content are permitted for publication: institution name, address, ownership structure, category, medical specialties, number of beds, clinic hours, and contact phone number (Note: The first six items must match exactly with those listed on the Medical Institution Practice License).
(2) Absolutely Prohibited Actions:
★Functional claims: such as "safe with no side effects," "cure completely," or "healing rate exceeds 90%";
★Use of Endorsers: Patients and doctors' images are prohibited as endorsements (including before-and-after treatment comparison photos);
★Comparative advertising: Belittling other organizations or exaggerating one's own strengths;
★Disguised advertising: Must not be published in the form of news, health education, free trials, or "recommendation-style sponsored content," among others;
★Creating Anxiety: Medical beauty ads are prohibited from associating appearance with labels like "success" or "poverty."
(3) Special Scenario Restrictions:
★Protection of Minors: Advertising is prohibited on campus, school buses, and in media aimed at minors.
★Sensitive Diseases: Radio and television are prohibited from airing advertisements for tumors, liver diseases, sexually transmitted infections, and similar conditions.
3. Standardizing Publication Practices and Channel Management
★Media compliance requirements: Public media advertisements must clearly indicate "ADVERTISING" to distinguish them from news content. For exclusive interviews and feature reports, the name of the organization may be mentioned, but no contact information is allowed—and these segments must be kept separate from advertising spaces.
★Third-party collaboration management: When entrusting advertising agencies, verify their business qualifications and clearly define responsibility for legal violations in the contract. Regularly monitor the partner’s publishing activities to prevent unauthorized modifications to ad materials.
(II) Establishment of a Compliance System for Medical Institutions
Medical advertising compliance is a component of a healthcare institution's overall compliance framework. Beyond medical advertising, other key areas of compliance risk that healthcare organizations must monitor throughout their operations include the quality and safety of medical services, the procurement and management of medical devices and pharmaceutical consumables, anti-corruption measures, information protection and data security, asset management, intellectual property rights, and environmental sustainability. To comprehensively identify and mitigate risks arising from hospital operations, healthcare institutions should also prioritize the development of a robust compliance management system. Drawing on the author's practical experience from leading a compliance management system-building and certification project at a public hospital, the following recommendations are offered for establishing an effective compliance framework in healthcare settings:
1. Top-Level Design and Organizational Structure
The establishment of a compliance management system requires strong leadership: building such a system is inherently the responsibility of top-level management, demanding deep involvement from healthcare organizations' leaders—especially the chief executive. Healthcare leaders must tailor the design of the compliance management structure to their organization's unique circumstances, seamlessly integrating it into the existing organizational framework. They should also consider appointing a Chief Compliance Officer to oversee and coordinate the responsibilities and reporting lines across the three-tier management system (Decision-Making Level – Management Level – Execution Level). Furthermore, all specific compliance initiatives must be strategically aligned and overseen by senior leaders, ensuring effective collaboration among their teams.
2. Comprehensive Risk Identification and Effective Implementation of Systems
The construction of a compliance system in healthcare institutions should focus on aligning with the institution’s own departmental structure and the operational management processes involved, enabling comprehensive risk identification across all areas. Based on experience, healthcare organizations should systematically assess compliance risks in at least the following key areas: medical service quality and safety, information and data security, third-party collaborations, anti-corruption measures, utilization of medical insurance funds, occupational safety and health, environmental protection, advertising and marketing, human resources and labor practices, intellectual property management, and asset management. Once identified, these risks should be evaluated and categorized into distinct risk levels, prompting tailored prevention and control strategies suited to each tier. Moreover, to effectively mitigate compliance risks, healthcare institutions must establish robust management systems and develop targeted process guidelines that seamlessly integrate compliance principles into daily business operations and administrative functions. In particular, compliance requirements should be embedded directly into critical operational steps—such as ensuring rigorous medical record-keeping standards for the proper use of insurance funds, upholding ethical practices during pharmaceutical and medical supply procurement, and adhering to transparent decision-making protocols for "major issues" requiring collective approval. This approach ensures that institutional policies are not merely theoretical but are actively implemented in real-world workflows, preventing the disconnect between policy and practice.
3. Compliance Culture and Continuous Improvement
Building a compliance system in healthcare institutions requires the active participation of everyone. It’s crucial to prioritize fostering a strong compliance mindset across the board. Healthcare organizations should tailor training programs according to the distinct responsibilities of medical staff and management teams, offering regular, tiered training sessions—such as focusing on clinical practice guidelines and integrity-building for clinicians, while emphasizing managerial skills and processes for administrative personnel. By setting clear compliance goals and linking these objectives directly to performance evaluations and incentives, institutions can reinforce accountability, ensure that compliance requirements are effectively implemented, and ultimately strengthen both individual and organizational compliance awareness and culture.
Building a compliance system in healthcare institutions is inseparable from continuous improvement. As healthcare organizations operate their compliance frameworks, both internal and external environments can shift at any time—such as the introduction of new policies and regulations. This underscores the need for healthcare providers to stay updated on the specifics of these evolving rules, ensuring that operational management stays well within regulatory boundaries and avoids crossing critical policy thresholds. Moreover, in day-to-day operations and management, healthcare institutions should adopt the "PDCA" (Plan-Do-Check-Act) management cycle to establish a dynamic monitoring mechanism. Regularly conducting compliance risk identification and assessments is essential, while promptly addressing any violations by implementing a closed-loop system: "Violation-Repair-Tracing."
Summary
The "Guidelines for the Supervision of Medical Advertising" establish a robust framework featuring "tiered regulation, four categories of prohibitions, and collaborative governance," designed to effectively target and curb illegal medical advertising. Empirical research highlights that the medical aesthetics, dental care sectors, and online advertising platforms are hotspots for violations in medical advertising. Common issues include advertisements published without prior review and the use of prohibited content. High-profile cases serve as cautionary tales for healthcare providers, emphasizing the need to avoid absolute language in medical ads, ensure strict adherence to regulatory procedures, and steer clear of marketing strategies that exploit concerns about physical appearance. Healthcare institutions must strengthen pre-publication reviews of medical ads, strictly follow the "negative list" of prohibited content, and carefully select compliant distribution channels. Moreover, they should integrate medical advertising compliance into their broader compliance systems, adopting a top-down approach that includes comprehensive risk identification across all areas, along with systematic process controls. Complementing these measures are efforts to foster a culture of compliance and drive continuous improvement, ultimately enabling healthcare organizations to achieve long-term, sustainable compliance management. By prioritizing compliance, institutions can build trust within the industry, safeguard patient rights, enhance the quality of medical services, and promote the healthy development of the healthcare sector.
Note:
[1] Article 75 of the "Provisions on the Standards for Filing and Prosecuting Criminal Cases under the Jurisdiction of Public Security Organs (II)."
[2] The relevant case data was sourced from a database of administrative penalties for medical advertising (searched by date for the most recent 100 administrative penalty cases related to medical ads), with the search conducted on June 11, 2025.
[3] Article 63 of the Administrative Penalty Law states: "When an administrative agency intends to impose an administrative penalty involving a substantial fine, it must inform the party concerned of their right to request a hearing."
Author Introduction
Su Zhongming
Beijing Xinglai Law Firm
Director of the Compliance Business Center
Bachelor of Laws from Jilin University, Master of International Law. As one of the first batch of corporate compliance professionals certified at the senior level, I have over 10 years of experience in legal affairs, asset management, and risk control & compliance across industries such as guarantee services and financial leasing. My expertise spans areas like corporate investment and financing due diligence, hands-on operations and risk management in financial leasing, and contract administration. Since joining Beijing Xinglai Law Firm, I have successfully led numerous projects focused on compliance systems for state-owned enterprises, central government-affiliated entities, public institutions, and private companies. Additionally, I’ve spearheaded the team that completed the Jiaxing City Compliance Demonstration Zone project. I’ve also been frequently invited to share my insights and conduct workshops at prestigious academic platforms, including Renmin University of China’s School of Law “Corporate Compliance Case Study Seminar” and the Beijing Normal University Education Training Center.
Business areas:
Corporate compliance management and critical risk emergency response, corporate mergers and acquisitions, investment and financing due diligence, as well as corporate civil and commercial disputes.
Li Zeyu
Beijing Xinglai Law Firm
Senior Consultant
Bachelor of Laws from Wuhan University, Master of Economic Law from Peking University. With over ten years of experience in legal and compliance management within the investment and financing sector, Mr. Li has focused throughout his career on private equity fund management, equity investments, cybersecurity and data compliance, antitrust law, and corporate governance compliance. He has been deeply involved in the full lifecycle of numerous private equity funds and M&A/restructuring projects, providing comprehensive legal support that includes legal due diligence, transaction structure design, post-investment management, and risk control. To date, he has contributed to projects totaling over 10 billion yuan in value, serving a diverse range of clients—including well-known private equity firms, leading technology companies, and major manufacturing enterprises. Mr. Li possesses a keen business acumen and exceptional communication and coordination skills, adept at integrating specialized financial and investment legal expertise with the unique industry characteristics of each project to effectively address clients' complex legal challenges.
Business areas:
Private equity fund management, equity investment, cybersecurity and data compliance, corporate governance, and antitrust compliance, among others.

Beijing Headquarters Address: Floor 17, East Section, China Resources Building, No. 8 Jianguomen North Avenue, Dongcheng District, Beijing
Wuhan Branch Office Address: Room 1001, 10th Floor, Huangpu International Center, Jiang'an District, Wuhan City, Hubei Province
Edited and Layouted by: Wang Xin
Review: Management Committee

Related News





